2025.10.06
EpiSensA Skin Sensitization Test Starts
― Advancing Ethical and Scientific Safety Evaluation through Alternatives to Animal Testing ―
We are pleased to announce the launch of our contract testing services for the in vitro skin sensitization test “EpiSensA”, in compliance with OECD Test Guideline 442D (OECD TG442D).
This test utilizes the human-derived 3D cultured skin model “LabCyte” as an alternative to animal testing. Compared with conventional evaluation methods, it enables a more ethical and scientifically sound assessment of skin sensitization (allergic potential).
The EpiSensA test is applicable to a wide range of samples, including cosmetic and quasi-drug ingredients, and serves as an effective tool for risk assessment and safety screening at the early stages of product development.
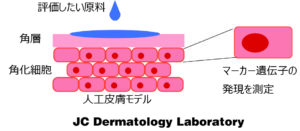
【Features of EpiSensA】
-
Reliable testing method compliant with OECD TG442D
-
High concordance with animal testing results, ensuring scientific validity
-
Applicable to both water-soluble and oil-soluble substances
-
Employs the artificial skin model “LabCyte”, which closely mimics the structure of human skin
-
Capable of evaluating the early phase of sensitization (Key Event 2), enabling earlier risk identification
【Additional Tests Available】
The following OECD guideline-compliant safety tests can also be conducted in combination:
-
Skin Irritation Test (OECD TG439)
-
Eye Irritation Test (OECD TG491)
By combining these tests, it is possible to comprehensively evaluate the effects of test substances on both skin and eyes.
We will continue to enhance our testing systems and expand our contract testing services in accordance with international standards, supporting our clients in achieving scientific and ethical product development.
For more information regarding test details, schedules, or service requests, please feel free to contact us.

 contact
contact